What does the phrase "pressurized container" mean? Explain using the concepts from previous lessons (e.g., particles, volume, number of particles). (min. 2 sentences)
Overview
This lesson asks the students to connect all their previous gas particle investigations. In contrast to previous lessons, they do not conduct any model-based explorations. Instead, they work on connecting the three gas equations they developed in the previous lesson and they try to apply those models to a real-world gas-related problem. They are then introduced to the Ideal Gas law, PV = nRT, which is a combination of the three models.
Standards
Next Generation Science Standards
- Physical Science
- [HS-PS2] Motion and Stability: Forces and Interactions
- NGSS Crosscutting Concept
- Patterns
- Systems
- Structure and Function
- NGSS Practice
- Analyzing Data
- Constructing Explanations, Designing Solutions
- Asking Questions, Defining Problems
- Using Models
- Arguing from Evidence
- Conducting Investigations
Computational Thinking in STEM
- Data Practices
- Analyzing Data
- Collecting Data
- Creating Data
- Manipulating Data
- Visualizing Data
- Modeling and Simulation Practices
- Assessing Computational Models
- Designing Computational Models
- Using Computational Models to Find and Test Solutions
- Using Computational Models to Understand a Concept
- Computational Problem Solving Practices
- Assessing Different Approaches/Solutions to a Problem
- Computer Programming
- Troubleshooting and Debugging
- Systems Thinking Practices
- Investigating a Complex System as a Whole
- Thinking in Levels
- Understanding the Relationships within a System
Credits
The 2019 version of this unit is developed by Umit Aslan (umitaslan@u.northwestern.edu) and Nicholas LaGrassa (nicholaslagrassa2023@u.northwestern.edu).
Acknowledgement
A majority of this unit is adopted from the earlier Connected Chemistry units developed by Uri Wilensky, Mike Stieff, Sharona Levy, and Michael Novak (see http://ccl.northwestern.edu/rp/mac/index.shtml for more details). Some elements are also taken from the Particulate Nature of the Matter unit developed by Corey Brady, Michael Novak, Nathan Holbert, and Firat Soylu (see http://ccl.northwestern.edu/rp/modelsim/index.shtml for more details).
We also thank undergraduate research assistants Aimee Moses, Carson Rogge, Sumit Chandra, and Mitchell Estberg for their contributions.
Activities
- 1. Let's think about the air duster can again
- 2. Putting the pieces together: developing an ideal gas equation
- 3. Let's talk about our ideal gas theories
- 4. The ideal gas law: making the theory work
Student Directions and Resources

- We observed a macro-level gas phenomenon (air duster can) and we tried to reason about the underlying micro-level events such as:
- What is a gas made up of?
- How do gas particles move in space?
- How do gas particles interact with each other and the walls of the container?
- We tried to qualitatively understand how micro-level interactions among gas particles lead to an important macro-level property: pressure.
- What is kinetic molecular theory?
- What is an ideal gas?
- We qualitatively and quantitatively explored the relationship between three variables and gas pressure: number of particles, gas temperature, container volume.
- How can we use computational models to study the behavior of gasses?
- How can we conduct computational experiments to: collect data, analyze data, and develop mathematical models?
- How do we validate our mathematical models?
- In what ways our mathematical models help us reason about real-world phenomena that involves gas pressure?



In this final lesson, we will reflect on our hard work and try to put together all three mathematical models, as well as our understanding of the behavior of gas particles, to develop a coherent theory of ideal gases.
1. Let's think about the air duster can again
We started this unit with an exploration on how the "air duster can" works. Let's revisit the air duster can again, but this time, about another interesting aspect of air duster cans (and all other spray cans), the warnings:

Can we use the knowledge we constructed through previous 5 lessons to explain why we need these warning texts on spray cans?
Question 1.1
Question 1.2
Why are we advised to protect it from sunlight and not expose it to temperatures higher than 50 degrees Celsius (≈122 degrees Fahrenheit)? What would happen if we did so? (min 3 sentences)
Note: Try to explain in narrative form (e.g., first ... would happen, then eventually ... happen because, finally .... happen because ...).
Also note: Try to include both micro-level events and macro-level events.
Question 1.3

Question 1.4
Do you think your understanding of objects like air duster cans, spray bottles, and balloons change after this unit? If yes, briefly explain how? (min. 2 sentences)
2. Putting the pieces together: developing an ideal gas equation
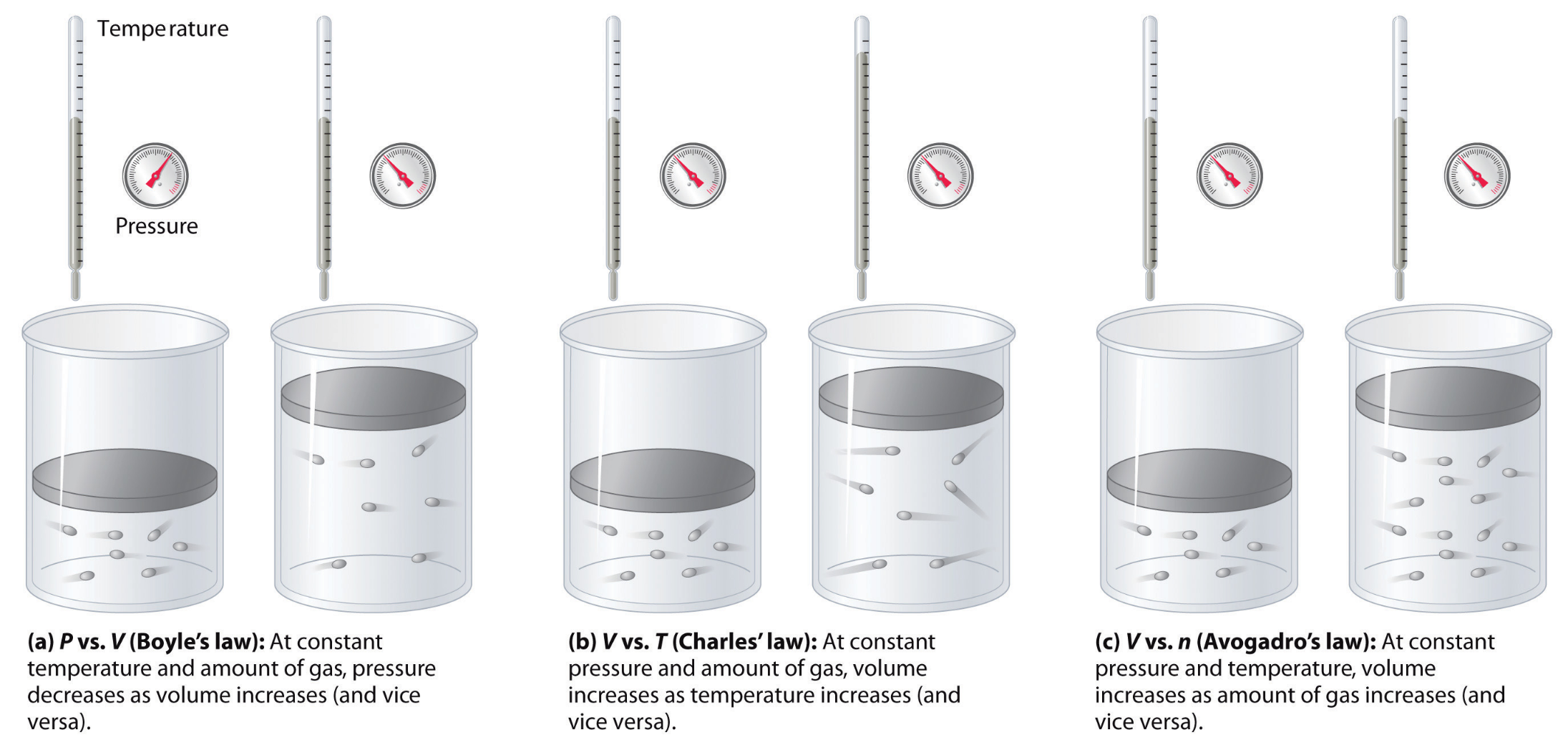
What we did in this unit was to re-develop three main gas theories using computational models and quantitative data analysis.
We defined the relationship between number of particles and pressure as:
- Qualitatively: As number of particles increases, pressure increases linearly (given that temperature and container volume are constant)
- Quantitatively: \((P)ressure = m_1 \times (N)umber \) , where \(m_1\) is a constant coefficient.
We defined the relationship between gas temperature and pressure as:
- Qualitatively: As gas temperature increases, pressure increases linearly (given that number of particles and container volume are constant)
- Quantitatively: \(P= m_2 \times (T)emperature \) , where \(m_2\) is a constant coefficient.
And, we defined the relationship between container volume and pressure as:
- Qualitatively: As gas temperature increases, pressure increases linearly (given that number of particles and container volume are constant)
- Quantitatively: \({P} = {m_3 \over (V)olume}\) , where \(m_3\) is a constant coefficient.
Our methodology and findings were analogous to three scientific discoveries made between 17th and 19th centuries:
However, as both the warning on the air duster can and the ballon-on-fire experiment showed, often times, we may not be able to explain gas-pressure related phenomena through just one variable. We need be combine our three theories and come up with one ideal gas theory. Let's try to do that!
Question 2.1
Can we put together our three findings in one coherent theory? Let's go ahead and give it a try!
First, write your combined theory verbally. Try to do it with just 1 coherent sentence.
Note: If you are having difficulty, you can write more than one sentence. It is perfectly fine.
Question 2.2
How about one mathematical equation that combines our three equations? Let's give it a try!
Alternative: If you are having difficulty in combining the equations, do not worry much. Either write the best equation you could come up with or write why you find this task particularly difficult.
3. Let's talk about our ideal gas theories
 Please set your computer aside briefly (do not close this page) and join the classroom discussion that your teacher is going to moderate.
Please set your computer aside briefly (do not close this page) and join the classroom discussion that your teacher is going to moderate.
Note: If your teacher did not initiate the discussion yet, you can start answering the first four questions below as you are waiting.
Question 3.1
If the discussion has not started yet, start answering the following question: Why even bother developing an ideal gas theory? In which situations such a mathematical theory might help us? (min 2. examples)
Question 3.2
Before moving on, please reflect on the classroom discussion briefly: (1) How did your verbal and mathematical ideal gas theory compared to the other groups' theories? (2) What were the similarities? (3) What were the differences? (min 2. sentences)
4. The ideal gas law: making the theory work
Let's review how we would put together our three theories mathematically:

\(P = m_1 \times N\) (Eq. 1)
\(P = m_2 \times T\) (Eq. 2)
\(P = {m_3 \over V}\) (Eq. 3)

\(P = {{(m_1 \times N) \times (m_2 \times T) \times (m_3)} \over V}\) (Eq. 4)
Step 3: We would slightly modify the equation by moving \(V\) to the other side of the equation and we would also get rid of the parentheses:
\(P \times V = {m_1 \times m_2 \times m_3 \times N \times T }\) (Eq. 5)
Finally, we would combine all three coefficients \((m_1 \times m_2 \times m_3)\)as one coefficient, which we can simply call as \(m_g\) or the ideal gas coefficient:
\(P \times V = {m_g \times N \times T }\) (Eq. 6)
Question 4.1
In the field of chemistry, our model is expressed slightly differently as \(P \times V = n \times R \times T\), where
- \(n\) corresponds to \(N\) or "number of particles" in our model, expressed in moles
- \(R\) corresponds to \(m\) or "gas coefficient" in our model, which is called the "universal gas constant."
- Its value is 0.08 L atm / mol K.
Answer the following chemistry problem using the ideal gas equation:
5 moles of nitrogen gas is in a 100 liter fixed cylinder at 300 Kelvin. What is the pressure of the gas?
Note: please determine the values of each of your independent variables (e.g., volume, temperature) and fill the data table below.
Question 4.2
Also answer the textbook question below:
Note: The temperature of air inside our lungs is approximately 37o C (273 + 37 = 310o K)
Question 4.3
Please describe how you solved the question above. Explain in steps. (min. 3 steps).
Such as:
- First, we did ....
- Then we did ....
- ....
Question 4.4
Optional question if you finished early: Answer the (b) part of the textbook question below. Also explain how you solved this problem.