Record all data from Investigation Part 1 in the data table below.

Overview
Students will dissolve five different ionic compounds in water to observe and measure the temperature difference before and after dissolving. Only endothermically dissolving ionic compounds are utilized here as the intention is for students to associate a compound’s bonds breaking with a temperature decrease as energy is required to break bonds. Kinetic energy from the water (the surroundings) is transferred to break bonds in the chemical system.
Students will use a spreadsheet in CODAP to systematically convert between various units. They will also observe how changing variables such as the amount of ionic compound used or the amount of water the ionic compound is dissolved in affects the temperature change.
Standards
Next Generation Science Standards
- Physical Science
- [HS-PS1-4] Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
- [HS-PS3-2] Develop and use models to illustrate that energy at the macroscopic scale can be accounted for as a combination of energy associated with the motions of particles (objects) and energy associated with the relative positions of particles (objects)
Computational Thinking in STEM
- Data Practices
- Collecting Data
- Creating Data
- Manipulating Data
- Visualizing Data
- Analyzing Data
- Modeling and Simulation Practices
- Assessing Computational Models
- Using Computational Models to Understand a Concept
- Systems Thinking Practices
- Communicating Information about a System
- Investigating a Complex System as a Whole
- Thinking in Levels
- Understanding the Relationships within a System
Activities
- 1. Investigation Part 1
- 2. Data Visualization
- 3. Automating Calculations Using CODAP
- 4. Investigation Part 2.A
- 5. Investigation Part 2.B
- 6. Analysis Questions
Student Directions and Resources
You will observe and record the temperature change when dissolving an ionic compound in water. Recall that an ionic compound is composed of cations and anions in specific proportions to create a neutral ionic compound.
You will need the following resources to complete this assignment.
1. Investigation Part 1
-
Record which ionic compound you are using in the table below.
-
Use a weighing boat to measure 1 gram of the ionic compound.
-
Use a graduated cylinder to measure 5 mL of water.
-
Record the initial temperature of the water.
-
In a small beaker, combine the water and ionic compound.
-
Place the temperature probe into the beaker and observe. Record the greatest difference in temperature from the starting temperature of the water/ionic compound mixture.
-
Dispose of water/ionic compound mixture in a sink and rinse out the beaker.
-
Repeat steps 1-7 with a total of five different ionic compounds.
Question 1.1
2. Data Visualization
In order to rank the five ionic compounds according to the temperature change in 5 mL of water, we will use an online data analysis platform called CODAP. Below, you will see a CODAP workbench. CODAP will allow us to visualize our data immediately upon entering it into the platform.
Let's begin with familiarizing ourselves with the CODAP environment:
 The platform is below these instructions. Notice an example ionic compound (NaCl) has been entered for you. Enter your data for the five ionic compounds you tested below this example.
The platform is below these instructions. Notice an example ionic compound (NaCl) has been entered for you. Enter your data for the five ionic compounds you tested below this example.- To better visualize the data, we can make a graph. In order to create a graph, drag the Ionic Compound variable to the x-axis of the plot as shown in the GIF on the right.
- Drag the ∆T/gram variable to the y-axis of the plot as shown in the GIF on the right.
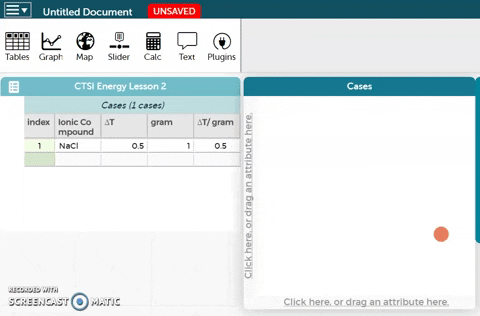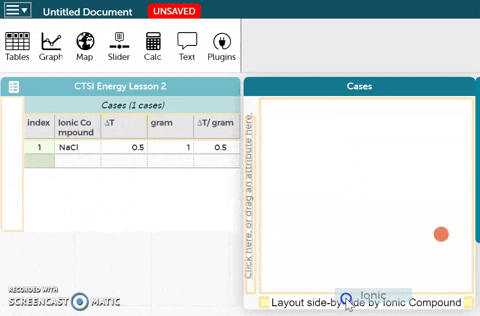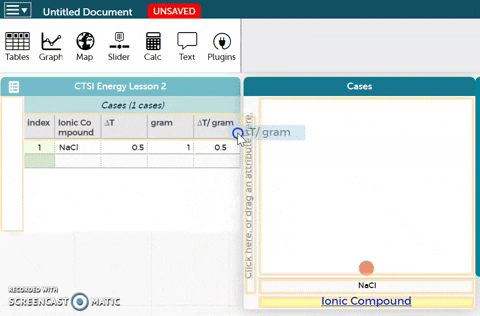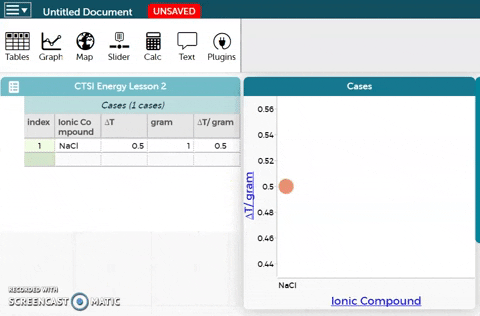
- Observe the resulting plot. Then answer the questions below the CODAP workbench.
Question 2.1
Rank the five ionic compounds according to the temperature change in 5 mL of water from greatest to least in the table below by expressing the value as ∆T/gram.
Question 2.2
Predict how your ionic compound rankings will look if we convert all temperature change data from ∆T/gram to ∆T/mole.
3. Automating Calculations Using CODAP
In order to convert our data from ∆T/gram to ∆T/mole, we need to find the molar mass of each compound. Then we can convert each ionic compound's mass from grams to moles. Finally, we would need to take our calculated temperature change and divide that by our moles, but that is so much work. What if we could make CODAP do some of that for us?
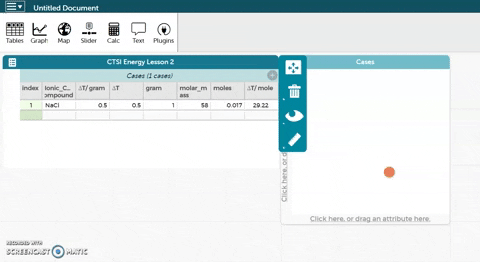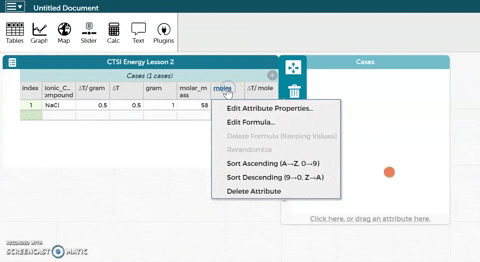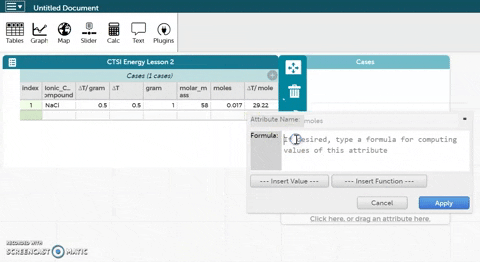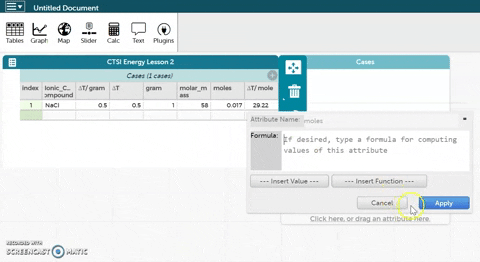 As before, the platform is below these instructions. The sodium chloride example has been expanded to include molar mass, moles, and ∆T/mole.
As before, the platform is below these instructions. The sodium chloride example has been expanded to include molar mass, moles, and ∆T/mole.- Calculate the molar mass for each ionic compound and enter it in the platform below.
- CODAP can now calculate moles for you if you provide it with the correct formula. Hold the cursor over moles as seen in the gif at right and click on Edit Formula. Use the asterisk (*) for multiplication and the slash (/) for division.
- Click Apply to close the formula editor. You should see values appear in the moles column.
- Now follow the same procedure to calculate values for the ∆T/mole column. Again to better visualize the data, we can make a graph.
- Drag the Ionic Compound variable to the x-axis of the plot.
- Drag the ∆T/mole variable to the y-axis of the plot.
- Observe the resulting plot. Then answer the questions below the CODAP workbench.
Question 3.1
How did converting the mass of the ionic compounds from grams to moles affect your ranking of the ionic compound's temperature changes? Describe specific changes for individual ionic compounds observed in the graphs in your answer.
Question 3.2
Why do you believe the changes you observed from the first graph to the second graph above occurred? Again be specific in your answer here.
Question 3.3
Why is it important to look at mole quantities of chemical substances?
4. Investigation Part 2.A
Last unit we explored how the amount of reactant will affect the amount of product produced. We want to expand upon this knowledge and explore how the amount of reactant affects other factors in chemical reaction. Read through the protocol below and determine which ionic compound you will use and the amounts of that compound you will use for each trial before starting your investigation.
Protocol Investigation 2.A – Vary the amount of ionic compound
- You will need to choose one ionic compound to test, from the ionic compounds tested in Investigation Part 1.
- You will need to vary the amount of ionic compound, staying within the 1-3 gram range.
- You will need to keep the amount of water constant, at 5 mL.
- You will need to record the initial temperature of each individually as well as the greatest difference in temperature from the starting temperature of the water/ionic compound mixture.
- Complete three trials.
Question 4.1
Record data from Investigation 2.A below.
Question 4.2
Describe how increasing the amount of the ionic compound affected your recorded temperature change.
Question 4.3
Why was it important to keep water at 5mL for Investigation 2.A?
5. Investigation Part 2.B
Use the same ionic compound from Investigation 2.A for Investigation 2.B. Read through the protocol below and determine the amounts of water you will use for each trial before starting your investigation.
Protocol Investigation 2.B – Vary the amount of water
- You will need to use the same ionic compound used in the first part of Investigation 2.
- You will need to vary the amount of water, staying within the 2-10 mL range.
- You will need to keep the amount of ionic compound constant, at 1 gram.
- You will need to record the initial temperature of each individually as well as the greatest difference in temperature from the starting temperature of the water/ionic compound mixture.
- Complete three trials.
Question 5.1
Record data from Investigation 2.B below.
Question 5.2
Describe how increasing the amount of water affected your recorded temperature change.
6. Analysis Questions
Question 6.1
Ionic bonds were broken when the solids were placed in water, was the temperature change that resulted expected or not? Why?
Question 6.2
Are these reactions exothermic or endothermic? Why?
